As global healthcare systems place increasing importance on transfusion safety, leukoreduction filters have become essential components in blood collection, processing, and transfusion. These filters are used to remove leukocytes (white blood cells) from blood components such as red blood cells (RBCs) and platelets. Their purpose? To minimize immunologic and non-immunologic transfusion reactions, prevent transmission of leukocyte-borne pathogens, and ensure compatibility with modern medical standards.
However, not all leukoreduction filters are created equal. For hospitals, blood banks, and medical device distributors, evaluating filter quality is critical—not only to meet regulatory compliance, but also to ensure consistent performance and patient safety. This article explores the Key Performance Indicators (KPIs) that define high-quality leukoreduction filters and guide informed purchasing decisions in the medical filtration market.
1. Leukocyte Removal Efficiency
The most critical performance indicator of a leukoreduction filter is its leukocyte removal rate, which quantifies how effectively the filter reduces white blood cells in a blood component.
Key Metrics:
· Target standard: Less than 1×10⁶ leukocytes per unit post-filtration (per AABB, EU, and WHO guidelines)
· Testing method: Flow cytometry or Nageotte hemocytometer
· Pass rate: ≥95% of filtered units should meet the removal threshold
Filters that consistently remove more than 99.99% of leukocytes ensure that the final product is free from residual white blood cells that could trigger immune responses or viral transmission.
2. Red Blood Cell or Platelet Recovery Rate
High leukocyte removal efficiency should not come at the expense of component recovery. A quality leukoreduction filter must preserve the therapeutic value of the blood product, especially RBCs and platelets.
KPI Benchmarks:
· Red Blood Cell recovery: ≥90%
· Platelet recovery: ≥80%
· Hemoglobin retention: Minimal loss (<5%) post-filtration
Superior filters achieve optimal leukocyte reduction while retaining the majority of red cells or platelets, maintaining hematocrit levels and clinical effectiveness.
3. Filtration Time / Flow Rate
In high-demand hospital and blood bank environments, time is a critical factor. The filtration time, or how long it takes to process one unit of blood through the filter, plays a significant role in operational efficiency.
Ideal Parameters:
· RBC filter flow time: <10 minutes per unit
· Platelet filter flow time: <5 minutes per unit
· No need for excessive gravity pressure or manual intervention
Inconsistent or slow flow times can indicate poor membrane design or clogging risks. High-quality filters maintain stable, predictable flow rates across a wide temperature range (typically 20–24°C).
4. Filter Integrity and Shelf Life
A leukoreduction filter must maintain performance over its storage life and under varying conditions. This is especially important for international procurement and inventory management.
Quality Indicators:
· Shelf life: ≥2–3 years under standard storage conditions
· Integrity: No membrane leakage, filter bypass, or structural defects
· Sterility: Gamma irradiation or EO sterilized, validated to ISO 11137 standards
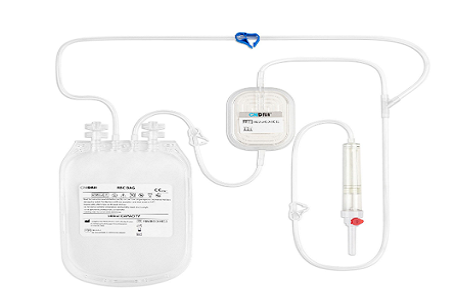
5. Compatibility with Blood Bag Systems
In practice, leukoreduction filters are often integrated into closed blood bag systems to maintain sterility and streamline processing. As such, compatibility with standard or custom blood collection systems is another important KPI.
Technical Features:
· Universal connectors (DIN, ISO compliant)
· Integrated in-line filters for pre- or post-storage filtration
· No additional handling required for connection/disconnection
Hospitals and distributors prefer filter systems that integrate seamlessly into their existing workflow, reducing labor, contamination risk, and processing time.
6. Residual Cytokine Reduction
Leukocytes not only carry viruses like CMV but also secrete cytokines, which can accumulate during storage and trigger febrile non-hemolytic transfusion reactions. Some advanced filters are engineered to reduce cytokines in addition to leukocytes.
Relevant KPIs:
· Reduction of IL-1, IL-6, and TNF-α: >50% in filtered units
· Assessment via ELISA or similar assays
· Clinical correlation with reduced febrile reactions
While not a standard requirement, this is a premium feature in high-performance filters, offering added safety for sensitive recipients such as cancer or transplant patients.
7. Filtration Mode and Usability
From a user perspective, ease of use and error prevention are important indicators of quality. Filters should offer intuitive handling, minimal manual steps, and visual cues to ensure correct operation.
Usability KPIs:
· Filtration mode: Gravity-driven, no pump required
· Priming needed: No or minimal priming required
· User errors: Low risk of reverse filtration or improper flow
· Visual indicators: Filter fill levels, clamp indicators, and clear labeling
Medical professionals, particularly in high-turnover environments, prefer filters that are simple and fast to operate while maintaining consistent outcomes.
8. Regulatory Certification and Compliance
A filter’s quality is also defined by its regulatory status, which assures compliance with international safety and manufacturing standards.
Required Certifications:
· CE marking (Europe)
· FDA 510(k) approval (U.S.)
· ISO 13485 (Quality management for medical devices)
· ISO 3826 compliance for blood bag systems
Filters without certifications often fail procurement standards, especially for public tenders or hospital systems bound by regulatory frameworks.
9. Batch Consistency and QA Documentation
Medical buyers and lab professionals also value filters that offer batch-to-batch consistency, minimizing performance deviation and ensuring reproducibility in clinical settings.
What to Look For:
· Certificate of Analysis (COA) with each batch
· Lot traceability and batch release testing
· Documented filtration efficiency per lot
· Manufacturing under Good Manufacturing Practices (GMP)
Filters from facilities with strict QA protocols are more reliable, reducing risk of clinical failure or recalls.
10. Customer Support and After-Sales Service
While not a technical KPI, customer support and post-sales service are crucial for bulk buyers, especially distributors, blood centers, and OEM customers.
Service Expectations:
· Training and usage guidance
· Fast replacement in case of defects
· Support for regulatory filing (e.g., IFUs, technical files)
· Multilingual support for export markets
Manufacturers that provide detailed product support gain long-term loyalty from medical customers and avoid disruptions in supply chains.
Conclusion: Why KPIs Matter in Filter Procurement
For healthcare providers, government tenders, and medical device distributors, selecting a high-quality leukoreduction filter is not just about price — it's about performance, compliance, and trust. By focusing on key performance indicators such as leukocyte removal rate, flow time, component recovery, and regulatory approval, buyers can make data-driven decisions that ensure both patient safety and supply chain reliability.
Whether you’re sourcing filters for blood bag manufacturing, supplying a hospital network, or seeking a private label/OEM filtration solution, keeping these KPIs in mind will help you identify products that meet today’s rigorous clinical and regulatory expectations.
For more information about DaJiMed leukoreduction filters and how they can enhance your blood filtration processes, contact us today. Let us help you improve blood transfusion safety with our premium filtration technology.
Copyright © Guangzhou DaJi Medical Science and Technology Co., Ltd. All Rights Reserved Sitemap | Powered by 