In recent years, the global healthcare industry has intensified its focus on enhancing the safety and quality of blood transfusions. One of the most significant advancements in this area is leukocyte removal, also known as leukoreduction — a process that removes white blood cells from blood components before transfusion.
With emerging clinical evidence, updated regulatory standards, and technological innovations, blood centers worldwide are now adapting to revised guidelines that emphasize universal leukoreduction practices.
This article provides an overview of the latest international recommendations, explains their clinical implications, and outlines practical strategies for blood centers to implement these new standards effectively.
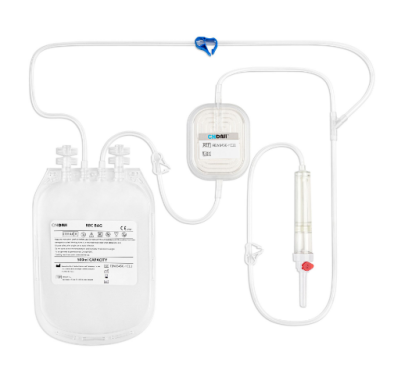
Leukocytes (white blood cells) present in donor blood can cause several adverse effects in recipients, including:
Febrile non-hemolytic transfusion reactions (FNHTRs)
Alloimmunization to human leukocyte antigens (HLA)
Transmission of leukocyte-associated viruses such as cytomegalovirus (CMV)
Immunosuppressive effects in immunocompromised patients
Numerous peer-reviewed studies published in leading journals such as The Lancet, Transfusion, and Blood have demonstrated that pre-storage leukoreduction significantly reduces these risks, making it a cornerstone of modern transfusion medicine.
Leading health organizations, including AABB (formerly the American Association of Blood Banks), the European Directorate for the Quality of Medicines & HealthCare (EDQM), and national regulatory bodies, now recommend or mandate universal pre-storage leukoreduction.
Key requirements include:
· Leukoreduction must occur within 48 hours post-collection
· Filters must achieve a residual leukocyte count of <1 × 10⁶ per unit
· Applies to red blood cell concentrates, platelets, and plasma components where indicated
This shift reflects a growing consensus that leukoreduction should not be limited to high-risk patient groups but applied universally to enhance overall transfusion safety.
To ensure consistent performance and compliance, blood centers are now required to implement rigorous quality assurance protocols, including:
· Routine leukocyte count testing of filtered units
· Documentation of filter validation data
· Use of certified leukoreduction filters compliant with ISO 13485, CE, or FDA standards
These measures help maintain product integrity and support traceability in case of audits or investigations.
While leukoreduction was historically targeted at specific high-risk populations, the latest guidelines advocate broader application:
· Universal leukoreduction for all red blood cell and platelet transfusions
· Priority implementation for:
· Immunocompromised patients
· Oncology and transplant recipients
· Neonates and pediatric patients
This expansion aims to minimize preventable complications across diverse patient populations.
Selecting the right filtration system is critical for meeting guideline requirements and ensuring operational efficiency. Blood centers are advised to:
· Source filters from reputable manufacturers with global certifications
· Require batch-specific test reports from suppliers
· Consider filters with:
· High flow rates
· Minimal red blood cell loss
· Compatibility with automated processing systems
Collaboration with qualified suppliers ensures long-term compliance and reliability.
Region | Regulatory Body | Key Requirements |
United States | AABB | Universal leukoreduction strongly recommended; strict QC protocols |
European Union | EDQM | Mandatory leukoreduction; defined residual leukocyte limits |
Japan | Japanese Red Cross Society | National standard for nearly all transfusions |
Australia | Australian Red Cross Blood Service | Universal leukoreduction policy in place |
Many countries have reported a significant decline in transfusion-related adverse events following the adoption of these policies.
Implementing the latest leukoreduction guidelines offers dual advantages — enhanced patient outcomes and operational efficiency for blood centers:
Reduced incidence of FNHTRs and HLA alloimmunization
Improved hospital satisfaction and institutional reputation
Compliance with international export standards for blood products
Lower risk of legal liability associated with transfusion complications
Better integration with automated and digital blood processing workflows
By adopting standardized leukoreduction protocols, blood centers position themselves as leaders in safe, modern transfusion practices.
To meet evolving standards, selecting the right filtration technology is essential. When evaluating leukoreduction filters, blood centers should consider the following criteria:
Evaluation Criteria | Description |
Filtration Efficiency | Verified leukocyte removal rate (<1 × 10⁶/unit) |
Component Compatibility | Suitable for RBCs, platelets, plasma |
Flow Rate & Processing Time | Optimized for high-throughput operations |
Certifications | CE, FDA, ISO 13485 |
Clinical Performance Data | Published evidence supporting efficacy and safety |
Customization Options | OEM/ODM capabilities for tailored solutions |
Choosing filters from trusted manufacturers ensures both compliance and long-term operational success.
As the global healthcare community continues to prioritize transfusion safety, blood centers must align with the latest leukoreduction guidelines to meet regulatory expectations and safeguard patient well-being.
Investing in advanced filtration technologies, implementing robust quality control systems, and partnering with certified suppliers are essential steps toward achieving compliance and maintaining excellence in blood component processing.
At DaJiMed, we are committed to providing high-performance leukoreduction filters and customized filtration solutions designed to help blood centers around the world meet the highest standards of safety and efficiency.
Ready to Upgrade Your Leukoreduction Process?
Contact DaJiMed today for:
· Filter selection guidance
· Technical documentation and compliance support
· OEM/ODM services
· Global logistics and customized packaging options
Partner with DaJiMed — your trusted expert in blood filtration innovation.
Copyright © Guangzhou DaJi Medical Science and Technology Co., Ltd. All Rights Reserved Sitemap | Powered by 